Surgical resection remains the most widely used curative approach for hepatocellular carcinoma (HCC), yet the postresection recurrence rate can be as high as 50%-70%. Numerous studies have explored adjuvant therapies for HCC, from the STORM trial in 2015 to the recent...
Month: April 2022
This Radiation Therapy Outperformed TACE in Liver Cancer
A meta-analysis found that patients with early and intermediate-stage hepatocellular carcinoma who received external beam radiation therapy (EBRT) demonstrated better local control and progression-free survival than those who received transarterial chemoembolization...
Adjuvant chemoradiation combined with immunotherapy for patients with high-risk resectable extrahepatic cholangiocarcinoma and gallbladder cancer: A phase II, multicenter, randomized controlled trial (ACCORD trial)
Adjuvant treatments specifically for resectable ECC and GBC patients are scare and adjuvant chemotherapy alone delivers limited efficacy. Immunotherapy and radiotherapy are potential effective treatments and both of them may synergize with chemotherapy. Learn...
Contrast-Enhanced Ultrasound May Improve LI-RADS Assessment of High-Risk Indeterminate Liver Lesions
Emerging research suggests that contrast-enhanced ultrasound (CEUS) may lead to more definitive detection of hepatocellular carcinoma (HCC) than magnetic resonance imaging (MRI) or computed tomography (CT). Learn More
What is Proton Therapy?
Zanidatamab exhibited durable antitumor activity among patients with previously treated advanced HER2-amplified biliary tract cancer, according to results of the phase 2B HERIZON-BTC-01 study presented at ASCO Annual Meeting. Learn More
The #HOPE4LIVER Single-Arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors
Histotripsy is a nonthermal, nonionizing, noninvasive, focused US technique that relies on cavitation for mechanical tissue breakdown at the focal point. Preclinical data have shown its safety and technical success in the ablation of liver tumors. Learn More
Zanidatamab exhibits ‘promising’ efficacy in advanced HER2-positive biliary tract cancer
Zanidatamab exhibited durable antitumor activity among patients with previously treated advanced HER2-amplified biliary tract cancer, according to results of the phase 2B HERIZON-BTC-01 study presented at ASCO Annual Meeting. Learn More
About the TheraBionic P1 device
Dr. Boris Pasche, MD, PhD explains how the TheraBionic P1 medical device works to treat cancer. The TheraBionic P1 device is currently FDA approved for treatment of adult patients with advanced hepatocellular carcinoma (HCC), the most common form of liver cancer, for...
Hepatocellular Carcinoma: Old and Emerging Therapeutic Targets
Liver cancer is one of the most difficult solid tumors to treat and is responsible for one-third of cancer-related deaths worldwide. In particular, the quest for effective therapeutic strategies for hepatocellular carcinoma, which often arises from a chronic...
The association of metabolic syndrome score trajectory patterns with risk of all cancer types
Metabolic syndrome (MetS) elevates cancer risk. However, a single MetS assessment does not fully reveal the long-term association with cancer. Inflammation, alongside MetS, could synergistically expedite both the onset and advancement of cancer. This study aims to...
Cholangiocarcinoma: Recent Advances in Molecular Pathobiology and Therapeutic Approaches
Cholangiocarcinoma (CCA) manifests as a complex interplay of genetic and environmental factors, necessitating personalized approaches. This review covers the various causes, risk factors, and molecular aspects of CCA in oncology. It explores current diagnostic methods...
IMFINZI® (durvalumab) plus transarterial chemoembolization (TACE) and bevacizumab reduced the risk of disease progression or death by 23% vs. TACE in liver cancer eligible for embolization
Positive results from the EMERALD-1 Phase III trial showed AstraZeneca’s IMFINZI® (durvalumab) in combination with TACE and bevacizumab demonstrated a statistically significant and clinically meaningful improvement in the primary endpoint of progression-free survival...
TriSalus developing novel therapeutic solutions for liver and pancreatic tumors
TriSalus Life Sciences (NASDAQ:TLSI) is addressing the mechanical and biologic barriers, such as high intratumoral pressure, within the tumor microenvironment (TME) that make it challenging to successfully treat liver and pancreatic tumors. Learn...
FDA Approves Merck’s KEYTRUDA® (pembrolizumab) Plus Gemcitabine and Cisplatin as Treatment for Patients With Locally Advanced Unresectable or Metastatic Biliary Tract Cancer
Merck (NYSE: MRK), known as MSD outside of the United States and Canada, announced that the U.S. Food and Drug Administration has approved KEYTRUDA, Merck’s anti-PD-1 therapy, in combination with gemcitabine and cisplatin, for the treatment of patients with locally...
Liver Cancer Awareness Month: Understanding and Treating HCC
Medical experts, Dr. Joan Culpepper-Morgan and Dr. Susanne G. Warner, touch upon treatment advancements for patients presenting with hepatocelluar carcinoma (HCC). In this article, both doctors cover a range of therapies that can be tailored and personalized to the...
Genentech’s Tecentriq Plus Avastin Reduced the Risk of Cancer Returning in People With Certain Types of Adjuvant Liver Cancer in a Phase III Study
In the first-ever positive Phase III trial in the adjuvant hepatocellular carcinoma (HCC) setting, Tecentriq plus Avastin reduced the risk of disease recurrence by 28%. Up to 80% of people with this type of HCC experience disease recurrence, at which point they are...
Hepatoblastoma
Hepatoblastoma is a very rare liver cancer (malignant tumor) that affects babies and toddlers ages 1 to 3. It’s treated with surgery. Rarely, hepatoblastoma may spread outside children’s livers. Hepatoblastoma may be “cured” with chemotherapy to shrink cancerous...
Genentech’s Tecentriq Plus Avastin Is the First Treatment Combination to Reduce the Risk of Cancer Returning in People With Certain Types of Early-Stage Liver Cancer in a Phase III Trial
Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced that the Phase III IMbrave050 study met its primary endpoint of recurrence-free survival (RFS) at the prespecified interim analysis. The study is evaluating Tecentriq® (atezolizumab) in...
Webinar: “The PERIO-02 Trial – Enabling Immunotherapy for Intrahepatic Cholangiocarcinoma”
In this webinar, Steven C. Katz, M.D., FACS, Chief Medical Officer at TriSalus Life Sciences, leads a review of the Pressure-Enabled Regional Immuno-Oncology (PERIO-02) clinical trial for adults with intrahepatic cholangiocarcinoma and hepatocellular carcinoma, the...
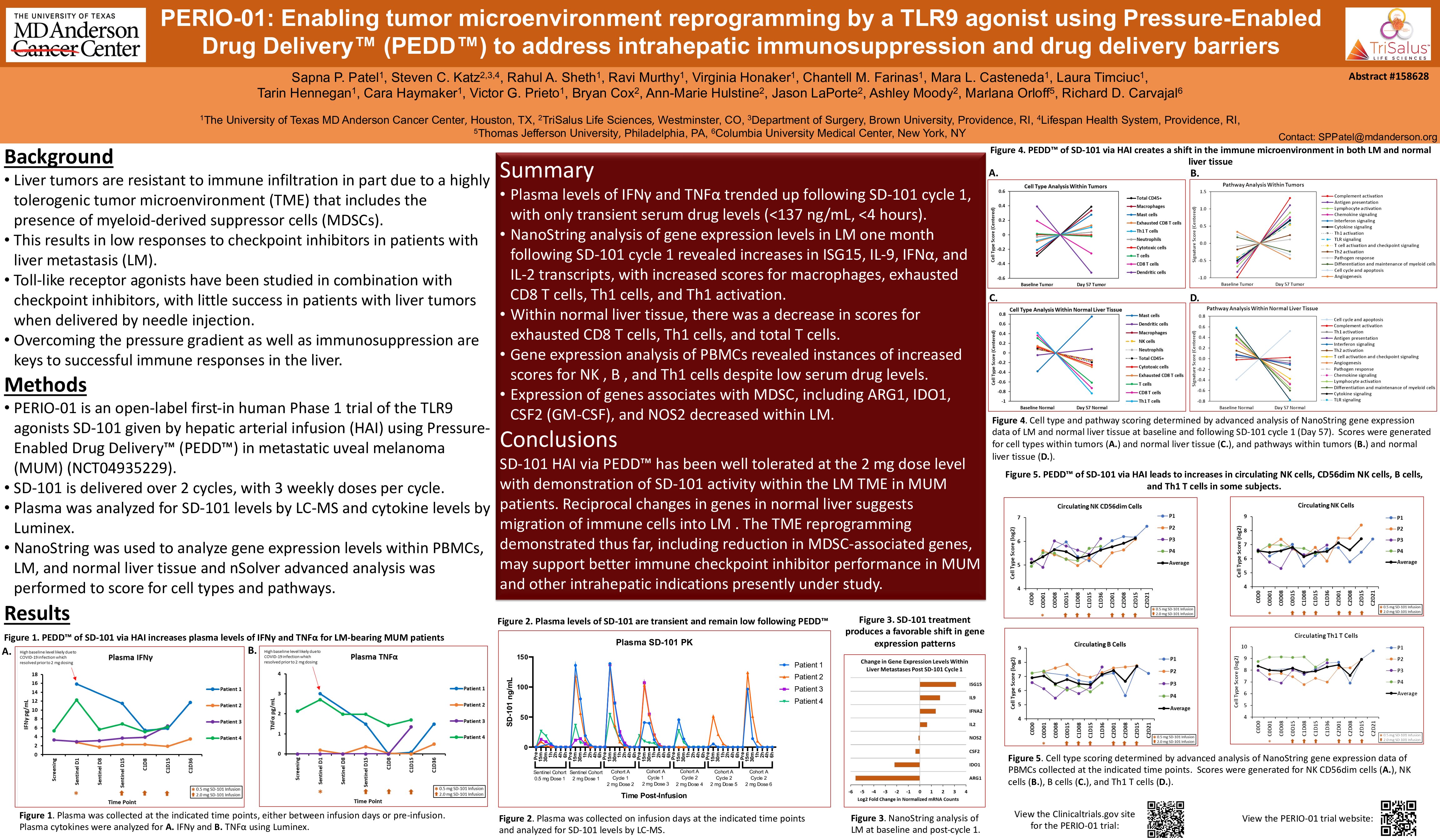
Early Data from PERIO-01 Presented at Recent Society for Immunotherapy of Cancer (STIC) Workshop
Dr. Sapna Patel from MD Anderson presented early data from the Pressure-Enabled Regional Immuno-Oncology-01 (PERIO-01) clinical trial at SITC’s Tumor Microenvironment: A Holistic Approach Workshop. Early findings suggest Pressure-Enabled Drug Delivery™ enabled SD-101...
A Cure in Sight and the Ocular Melanoma Foundation Collaborate to Host Webinar on PERIO-01 Clinical Trial for Uveal Melanoma with Liver Metastases
A Cure in Sight, in collaboration with the Ocular Melanoma Foundation, hosted an educational webinar on the Pressure-Enabled Regional Immuno-Oncology-01 (PERIO-01) clinical trial for uveal melanoma with liver metastases. This webinar featured insights from Drs....
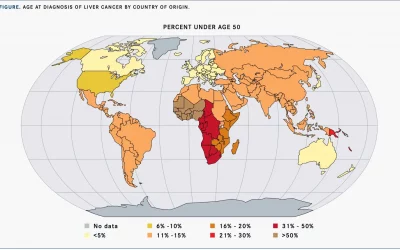
Achieving Health Equity in Liver Cancer
Primary liver cancer death rates vary by region, and much is still to be done in the area of health equity. Primary liver cancer is a leading cause of cancer-related illness and death worldwide. It is the sixth most-common cancer and the third most-common cause of...
Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial
Health related quality of life (HRQOL) for patients undergoing treatment for unresectable hepatocellular carcinoma is an important therapeutic consideration. The evidence of HRQOL benefits in clinically relevant domains support the use of lenvatinib compared with...
Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma
Hepatocellular carcinoma is a common cancer worldwide and a leading cause of cancer-related death. Although early-stage disease may be curable by resection, liver transplantation, or ablation, most patients present with unresectable disease and have a poor prognosis....